Articles
Clarifying Common Disinformation Regarding Colloidal Silver
Clarifying common disinformation regarding colloidal silver, which is readily pushed to prevent the average person from even considering the health benefits through the use of fear.
Whenever you make colloidal silver with electricity (electrolysis), you will always end up with both ionic silver and silver particles (colloids).
The specific ratio between them is largely governed on how you go about making it.
The first major scare tactic used to discourage consideration is that drinking colloidal silver will turn you blue -- a condition called argyria.
First in order to turn blue, you have to drink copious amounts of silver salts just like the famous guy Paul Karason (who did it on purpose), who is really the only person used to indicate this condition as it is very rare. Neither ionic silver nor silver colloids will turn you blue. You would have to purposely taint your colloidal silver process to produce silver salts in quantity, and it takes effort and time.
The reason silver salts can turn you blue is that when exposed to photons under the skin, they can develop just like old silver halide based photography. It's like giving yourself a tattoo from underneath your skin.
Second you will hear about silver toxicity or that silver is a heavy metal. This is not the case. Silver is a noble metal just like gold. They put gold in drinks all the time, and it passes through the body without issue.
Certain silver compounds can be troubling but neither ionic silver nor silver particles are.
Then there's the studies that show extremely large amounts of silver itself can be toxic. Well to be fair, in order to drink enough silver to reach these toxic levels, you would first die of too much water -- it would take nearly 500 gallons in a short period of time.
All silver ingested typically leaves the body within 24 to 48 hours.
Third it is common to point out that ionic silver will instantly bind to chlorine in the body and form silver chloride which they will say is an insoluble solid. In reality is has very low solubility in water, but it is not insoluble.
Arguments are made that since ionic silver will instantly bind with chlorine, it can't do anything good in the body and only silver particles are useful. They will then point out that most colloidal silver is actually ionic silver. Again, when you make colloidal silver, you will end up with both as it is not possible to make 100% one or the other.
Even if you could make 100% ionic silver, some of the ionic silver will be reduced to silver colloids through the photoelectric effect -- when the solution is exposed to light.
In any event, they will point out that you have chlorine in your stomach and in your blood stream, so ionic silver can't survive as it will readily turn into silver chloride.
Well chlorine in the stomach is already is bound to hydrogen as hydrochloric acid. Chlorine in the blood is typically already bound to potassium or sodium as typical salt. You do not have copious amounts of just chlorine sitting in the body waiting to bind to silver.
They make arguments that silver will take the chlorine from said sources with no understanding of chemistry.
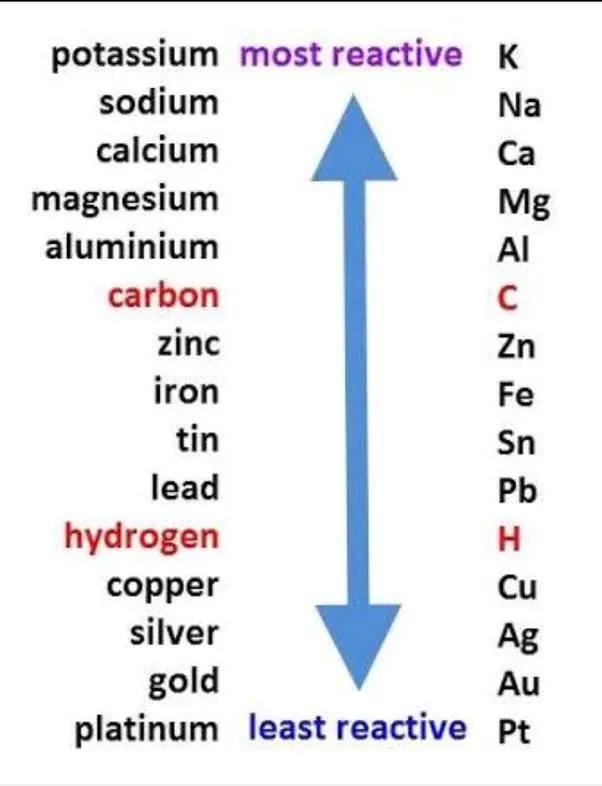
Silver is very low on the reactivity series, well below hydrogen, sodium, and potassium -- meaning the last 3 have stronger bonds.
Hydrochloric acid is typically used to clean silver, and if it reacted with the silver, it would eat the silver up and not be a very good cleaner.
What does dissolve silver? Silver metal dissolves in hot concentrated sulfuric acid and also dissolves in dilute or concentrated nitric acid, HNO3.
While the hospitals and medical websites tell you silver is not safe, they still use it to treat burn victims in the form of silver nitrate which is not a compound you would want to drink. In fact they still use silver nitrate drops in newborn baby's eyes to prevent blindness due to unforeseen infections.
A typical "What's good for us is not good for you" approach.
Finally, while this is not a scare tactic, it does qualify as poorly understood aspects of colloidal silver.
A TDS (Total Dissolved Solids) meter measures conductivity of a solution, and multiplies the voltage against a factor representing the totality of commonly dissolved solids (typically in tap water) to provide you with a PPM estimation.
Only the ionic silver portion is dissolved and conducts current. The factor used by a TDS meter is no where near accurate to represent only ionic silver. You can not measure the silver particle (colloid) content with a TDS meter.
A laser pointer used to look for the Tyndall effect (scattering of the laser beam so it becomes visible) can not measure ionic silver.
Ionic silver is fully dissolved in a solution and would not be able to interact with the photons. It can only show you when there is silver particles (colloids) in solution, and only when they are sufficiently large enough.
If you can see a laser line in your colloidal silver solution, your particles are not very small and thus will be less bio-available.
If you brew too long to keep adding silver particles, they will be forced to aggregate together into larger particles. You can only shove so much silver into water.
Once aggregated, they do not break back up. After about 30 ppm, they will be sufficiently large enough to absorb blue light, and the solution will turn gold. Continue to brew and eventually it will turn brown, then black.
Buying 250 PPM solutions and diluting them down is not the same -- again the particle size will have been forced to aggregate into very large particles.
All you need is between 10 to 20 ppm of clear colloidal silver solution which has been shown to be effective in disrupting the respiratory process of pathogenic factors.
Colloidal Silver is not an antibiotic. It is an antimicrobial. There is a difference.
Blessings
Our Mission
Elevate Your Wellness Journey and Take Control with Your Very Own Colloidal Silver.
Copyright© 2025 TechHolistix™ / Silver4Health™ | Privacy Policy | Terms & Conditions
DISCLAIMER: This website, including products, articles, and educational content is not intended to diagnose, cure, or prevent any disease. The information contained on this website is for general educational purposes only. This website does not provide medical advice.